
#Animate secondary structure vmd software
Results from peptide prediction software and molecular dynamics simulations confirmed that ascaphin-8 is an alpha-helical peptide. However, up to date, their principal molecular target and mechanism of action are unknown. We determined that Bucl8 is a component of a novel tetrapartite efflux pump, which confers FA resistance, fibrinogen binding, and optimal growth.Īscaphins are cationic antimicrobial peptides that have been shown to have potential in the treatment of infectious diseases caused by multi-drug resistant pathogens (MDR). We finally demonstrated that deletion of the bucl8 -locus drastically affects the growth of the mutant in L-broth. On the contrary, the Bucl8-associated pump did not confer resistance to a panel of clinically-relevant antimicrobials in Burkholderia and E. We furthermore showed that the putative Bucl8-associated pump expressed in the heterologous Escherichia coli host confers FA resistance. pseudomallei, while deletion of the entire bucl8 locus decreased the minimum inhibitory concentration of FA 4-fold in its isogenic mutant. We found exogenous FA induced bucl8 transcription over 80-fold in B. Using reverse transcriptase (RT)-qPCR, we defined the boundaries and transcriptional organization of the fusR-bucl8-fusCDE operon. The bucl8 gene is co-localized with downstream fusCDE genes encoding fusaric acid (FA) resistance, and with an upstream gene, designated as fusR, encoding a LysR-type transcriptional regulator. Bioinformatic analysis of the bucl8 gene locus revealed it resembles a classical efflux-pump operon. Molecular modeling and circular dichroism spectroscopy with a recombinant protein, corresponding to this extracellular CL-Ct portion of Bucl8, demonstrated that it adopts a collagen triple helix, whereas functional assays screening for Bucl8 ligands identified binding to fibrinogen. Unique to Bucl8, as compared to other outer membrane proteins, is the presence of an extended extracellular region containing a collagen-like (CL) domain and a non-collagenous C-terminus (Ct).
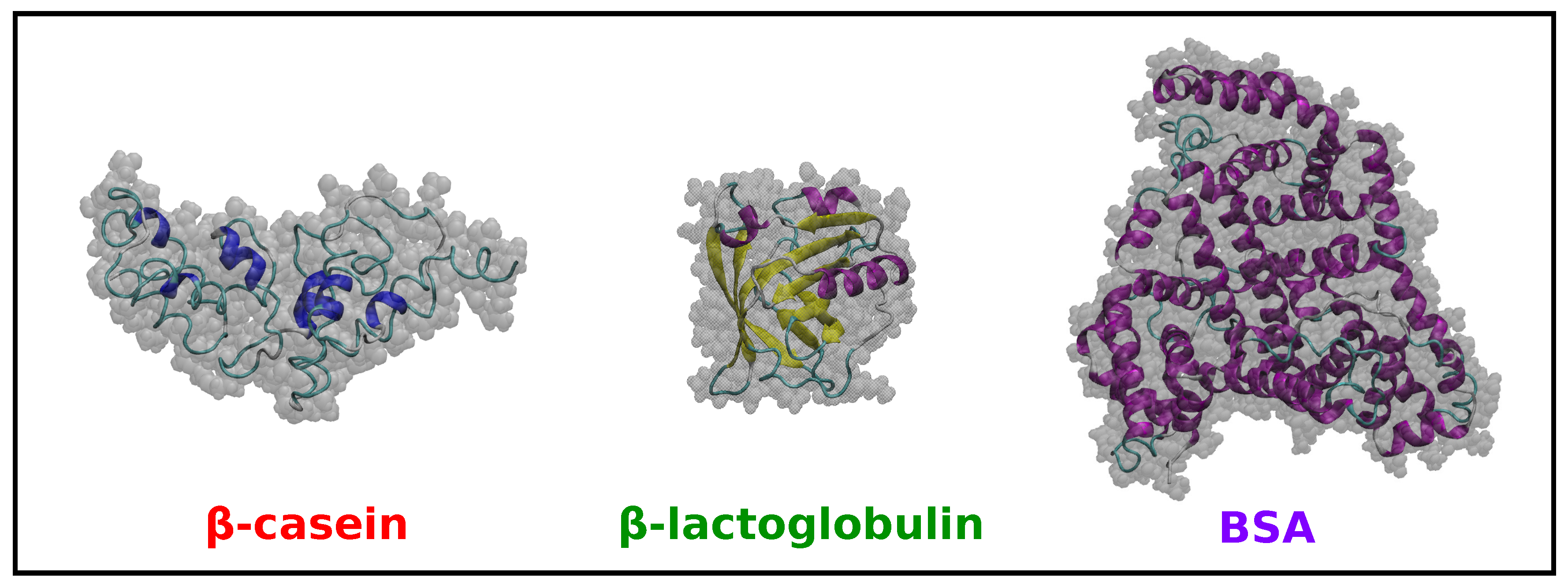
We hypothesize that Bucl8, which contains two predicted tandem outer membrane efflux pump domains, is a component of a putative efflux pump. Our laboratory previously identified in silico Burkholderia collagen-like protein 8 (Bucl8) in the hazardous pathogens Burkholderia pseudomallei and Burkholderia mallei. Bacterial efflux pumps are an important pathogenicity trait because they extrude a variety of xenobiotics.


 0 kommentar(er)
0 kommentar(er)
